Metallic bonding is the third type of bonding you need to know about.
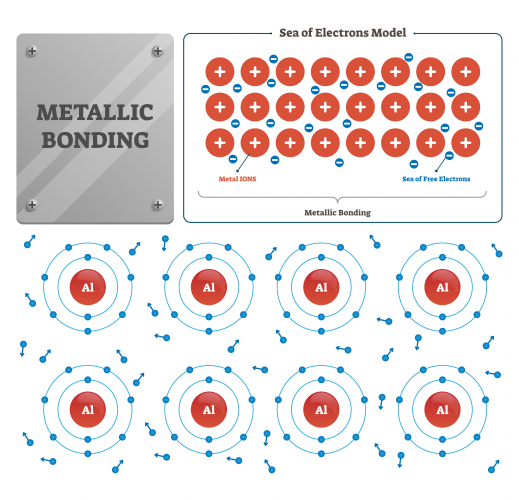
You can guess from the name that it's the type of bonding that happens between two metallic atoms. All the atoms in a piece of metal need to lose electrons, none will take any more electrons. The unwanted electrons move between the atoms - scientists talk about a 'sea of electrons' flowing between the metal ions.
Another word used to describe the electrons is delocalised. Something local is in a definite place, so delocalised means that something isn't in a definite place. The number of delocalised electrons depends on the electron structure of the atom. Atoms of Group 1 metals give off one electron, Group 2 metal atoms give off two, and so on. In this picture, the Al (aluminium) atoms have each put three electrons into the sea of electrons.
The atoms in a metal are held together by electrostatic attraction between the positive metal ions and the delocalised negative electrons. The metal ions are pulled tightly together in a regular repeating close-packed structure. Scientists call structures with regular repeats crystalline. Metal structures are crystalline, as are most ionic substances. The crystalline structure of gold looks like this:
.png)
We can explain a lot of the properties of metals by thinking about this model of metallic bonding. The delocalised electrons aren't attached to atoms, so they can move through the metal easily. This makes metals good conductors of electricity and heat, even when they are solid. Ionic compounds do not conduct electricity when they are solid - there are ions, but they cannot move. (Ionic compounds dissolved in water can conduct electricity, because the ions can move freely).
The electrostatic bonds between the metal ions and the delocalised electrons are hard to break, so metals are strong. However, it's easier to move layers of atoms over each other. This means that metals can easily be bent into new shapes - scientists call this being malleable. The combination of strength and malleability means that metals are easy to bend but hard to break. This makes them extremely useful for making things. The strength of the metallic bonds also explains why metals have high melting and boiling points - we need to add a lot of energy to the metal to break the bonds between the metal ions.
We can also explain some of the patterns in the properties of different metals by thinking about the sea of electrons. The more electrons each atom delocalises into the sea of electrons, the stronger the forces between the leftover ions will be. This means that Group 1 metals are much less strong than other metals. The more delocalised electrons there are, the better the electrical conductivity.
Thinking about metals shows why it's so useful to understand the electron structure of atoms.
Remember that all metal atoms are ejecting electrons, and that there's nowhere really for the electrons to go. Once you have that picture in your mind, most of the properties of metals make sense.
Now let's have a go at some questions.








