Once we know how atoms bond together in compounds, we can understand why the compounds have the properties they do. In this activity, you will learn what ionic compounds are like, and why.
In ionic compounds, metal atoms lose electrons to become positively charged ions, and non-metal atoms gain electrons to become negatively charged ions. Positive and negative charges attract each other, so we get ionic bonds holding the ions together. Positive attracts negative, negative attracts positive, and so on.
The only thing that stops the pattern is when you run out of atoms. Since the structure can have many, many millions of atoms in it, we call it a giant structure. Giant ionic structures are crystalline, because they have a repeating pattern.
.png)
In the picture above, there are equal numbers of Na (purple) and Cl (green) ions, so we can tell that the formula of the structure is NaCl.
Different people draw the same structures in slightly different ways. In a space-filling diagram (like the NaCl picture), we have a realistic picture of the size of the different ions and how they bond. Unfortunately, it's hard to see all the atoms, because some are hidden.
In a ball-and-stick model, we shrink the atoms (the balls) and link them by bonds (the sticks). We can then see more of the atoms more clearly through the gaps. To really visualise the structure, it's even better to look at a model or a computer animation. The next picture shows zinc sulfide, as a ball-and-stick model - zinc is blue, and sulfur is yellow.
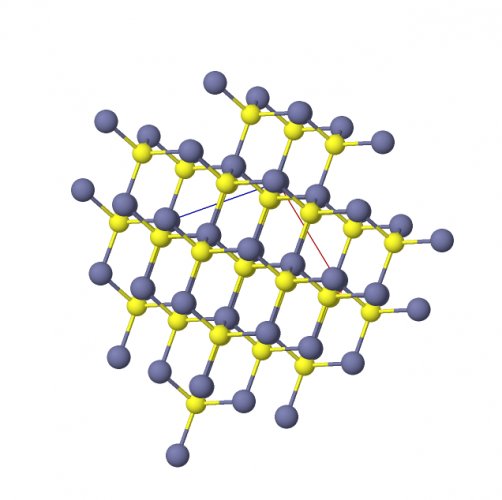
All ionic compounds have these properties:
They have high melting and boiling points: This is because there are strong ionic bonds in all directions. To break these bonds, large amounts of energy is needed, which comes from high temperatures.
Most ionic compounds dissolve in water: This happens because water weakens the electrostatic bonds holding positive and negative ions together.
Solid ionic compounds are insulators - they don't conduct electricity: Although the ions carry charge, their positions are fixed in the crystal structure, so they can't move.
Molten ionic compounds can conduct electricity: This is because the ions in a liquid can move. When they move, they carry electrical charge with them.
If ionic compounds dissolve in water, the resulting solution also conducts electricity.
When you think about giant ionic structures, remember that they are made of positive and negative ions.
The properties of giant ionic structures work because there are strong forces between the ions, which stop them moving.
However, we can use water to weaken those bonds, which makes a solution where the ions move around easily in the water.
Now let's move on to some questions.








