In everyday life, chemistry involves chemical reactions which we can see, such as cooking, and experiments at school using test tubes. However, chemical changes take place at particle level and there are millions, even billions of these involved in every chemical reaction.
Nanochemistry deals with individual particles, i.e. at atom level. It is possible to arrange atoms inside an element in a different way and create allotropes of the same element - for example, diamond, graphite and fullerenes are all allotropes of carbon.
Diamond is used in jewellery but because it is the hardest substance that exists, it is also used in the manufacturing and building industries for cutting-tools. Diamonds have a high melting point, do not conduct electricity and are insoluble in water. Diamonds used in jewellery are almost colourless, clear and shiny, but industrial ones are coloured and opaque. Each atom is held by covalent bonds to four other atoms which, in turn, are bonded to more and so on. They form a giant structure and the covalent bonds are incredibly strong, so the melting point is very high. The picture below shows a model of the structure of diamond:
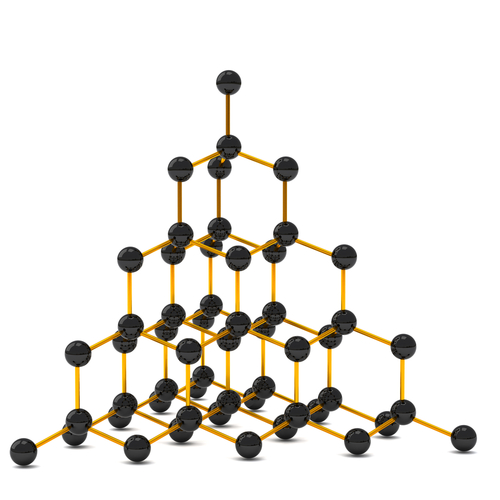
Graphite is also made of carbon but it is soft, opaque and black (but still shiny). It is also insoluble in water but conducts electricity very well. Its melting point is high, so it can be used to make electrodes for electrolysis. It is made of flat layers that are far apart which makes it slippery, so it can be used to make pencils. When you write with a pencil, some of the graphite slides off and leaves a mark on the paper. Look at the diagram below to see how different the structure of graphite is to that of diamond:
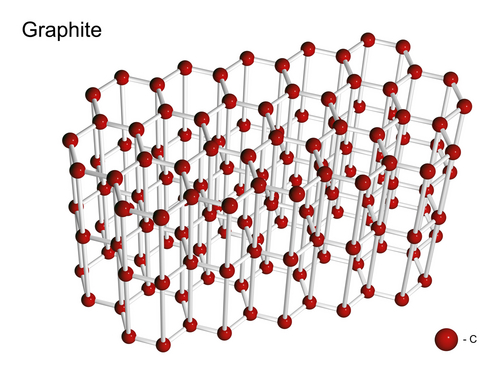
Fullerenes, also made of carbon, look very different to both graphite and diamond. Have a look at the structure of fullurenes:
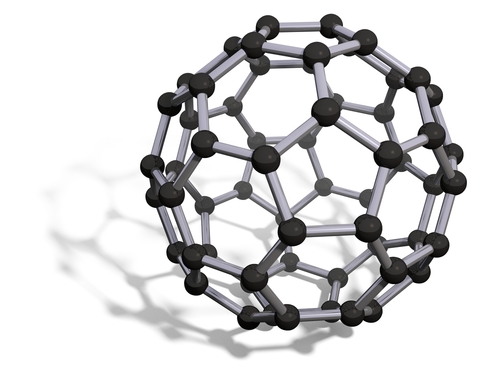
Fullerenes are black solids, insoluble in water but soluble in petrol. They are used to carry drug molecules around the body, and trap and remove dangerous substances from the body. Their nanotubes are used in catalyst systems.
Now it's time for some questions on this topic.








