EdPlace's GCSE Home Learning Chemistry Lesson: Electrolysis
Looking for short lessons to keep your child engaged and learning? Our experienced team of teachers have created English, maths and science lessons for the home, so your child can learn no matter where they are. And, as all activities are self-marked, you really can encourage your child to be an independent learner.
Get them started on the lesson below and then jump into our teacher-created activities to practice what they've learnt. We've recommended five to ensure they feel secure in their knowledge - 5-a-day helps keeps the learning loss at bay (or so we think!).
Are they keen to start practising straight away? Head to the bottom of the page to find the activities.
Now...onto the lesson!
Electrolysis: Splitting of Molten Ionic Compounds into Metals and Non-Metals
Electrolysis can be one of the trickiest subject areas that students will come across in their GCSE Chemistry course. For parents, the best way to support your child is to break down the topic into smaller parts. Many of the marks for this topic can be gained by learning basic rules, combining them and applying them in larger questions. This will boost your child’s confidence in Chemistry overall.
At EdPlace we’re surrounded by a team of experts who communicate these concepts with children on a day-to-day basis, and we’re ready to share their teaching gems with you. Follow the step-by-step approach below to make this problem half as tricky! We're confident if you follow this step by step approach your child will:
1) Understand how the basic principles of molten electrolysis work to split ionic compounds
2) Apply this to several examples of ionic compounds
3) Explain this process back to you (if they've really cracked it!)
Step 1: Understand the Scientific Terminology
Before we look at the process of electrolysis in detail there are multiple scientific terms your child will need to get to grips with. The good news is that apart from the few terms below most of the terminology used will be words your child is familiar with if they have studied the prior modules in Chemistry.
An electrolyte is an ionic compound that is to be broken down. Ionic compounds are made of metals and non-metals that are chemically bonded.
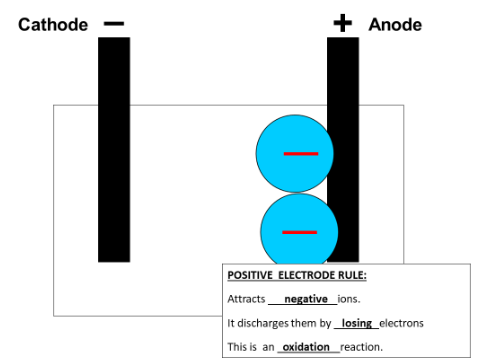
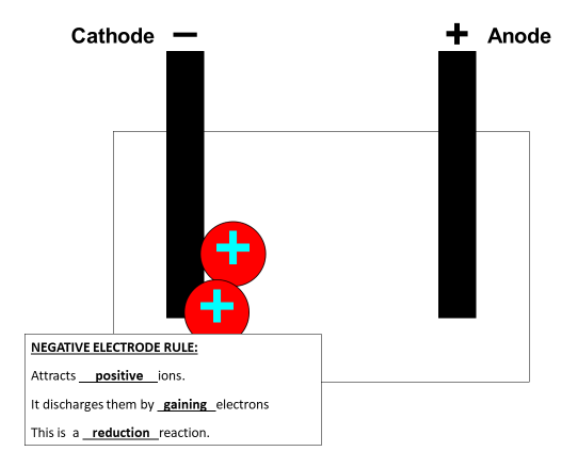
An anode is a positive electrode and a cathode is a negative electrode.
Oxidation is the loss of electrons and reduction is the gain of electrons.
Step 2: Recap
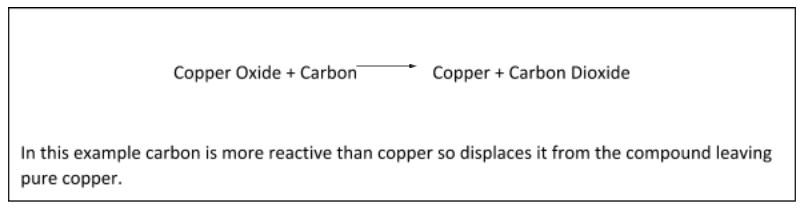
Only unreactive metals such as gold are found naturally. More reactive metals which include aluminium (the most used metal on the planet) are found naturally in ionic compounds bonded to a non-metal. We often call this a metal ore.
Less reactive metals:
Metals that are less reactive than carbon can be extracted using a displacement reaction. This is where the metal ore is reacted with carbon. The more reactive carbon displaces the metal from the ionic compound.
More reactive metals:
Metals more reactive than carbon will not get displaced and so we must use electrolysis to split them from the non-metal in their compound. Electrolysis relies on charges and electricity. Atoms which have a charge are called ions. Negative ions have gained extra negatively charged electrons. Positive ions have lost negatively charged electrons.
Step 3: Understand Electrolysis
There are two types of electrolysis. Electrolysis of molten electrolytes which is the focus of this lesson. Then, there is also electrolysis of electrolytes dissolved in solution which should be revised as a follow-up lesson. The process of electrolysis can be always divided into four stages:
Stage 1: Turning the ionic compound into an electrolyte
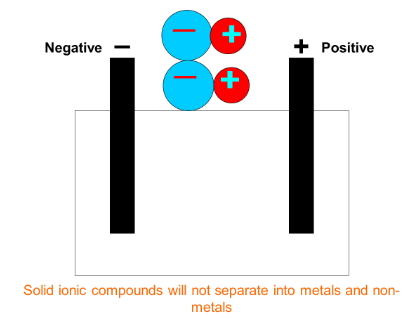
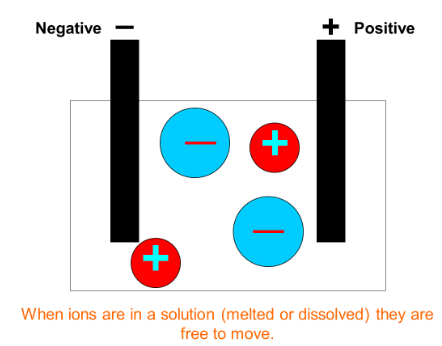
In order to separate the metal and non-metal atoms bonded together in an ionic compound, we must first break bonds holding the atoms together. This forms metal ions (positive) and non-metal ions (negative). For electrolysis to work ions must be in liquid form so they are free to move.
We can do this in one of two ways:
1) Some ionic compounds are insoluble so the only way we can split them is by melting them. An example of this would be aluminium oxide (Al2O3) which must be heated to very high temperatures to break the bonds allowing it to turn to liquid. This is the focus of this lesson.
2) Some ionic compounds are soluble which means they will simply dissolve in water. An example of this would be sodium chloride (NaCl) which can be dissolved in water to make the metal ion sodium (Na+) and the non-metal ion chloride (Cl-). This should be studied in another lesson.
Stage 2: Using electrodes to move ions apart.
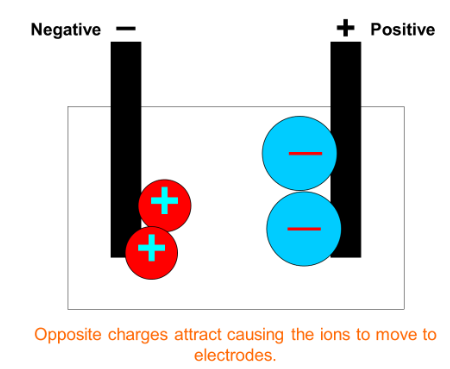
After stage 1 the aluminium ions in the molten electrolyte are still mixed with oxygen ions so they must be separated. To do this we put electrodes into the liquid electrolyte mixture and pass an electric current through the solution. There are two electrolytes; one is positive and the other is negative.
The non-metal ion (negatively charged) will move towards the positive electrode (anode) as opposite charges attract.
The metal ion (positive charged) will move towards the negative electrode (cathode) as opposite charges attract.
Stage 3: Oxidation of the non-metal ion at the positive electrode (anode).
The non-metal ion (negative) now attaches to the positive electrode and it is discharged to become a neutral non-metal atom.
Oxygen ions have a charge of -2 so are presented as O2-. This means to become an atom with no overall charge they must lose 2 negative electrons. This is an oxidation reaction (remember OIL RIG (Oxidation Is Loss).
Stage 4: Reduction of the metal ion at the negative electrode (cathode).
The metal ion (positive) now attaches to the negative electrode and it is discharged to become a neutral metal atom.
Aluminium ions have a charge of +3 so are presented as Al3+. This means to become an atom with no overall charge they must gain 3 extra negative electrons. This is a reduction reaction (remember OIL RIG (Reduction Is Gain).
Step 4: Apply Your Knowledge to New Examples
Look at the image below and have a go answering the following together.
a) Is copper (Cu2+) a metal or a non-metal and how do you know?
b) Is chlorine (Cl-) a metal or a non-metal and how do you know?
c) What two methods could be used to break the bonds in copper chloride (CuCl2) to separate it into copper ions (Cu2+) and chloride ions (Cl-)?
d) Which electrode will the copper ions (Cu2+) move towards when the electricity is turned on and why?
Stretch and challenge:
e) When it moves to the electrode what reaction will happen to the copper ion (Cu2+) and what does this mean?
f) When they move to the electrode what reaction will happen to the chlorine ion (Cl-) and what does this mean?
Step 5 - Putting it into Practice
Now, you’ve covered this together why not put this to the test and assign your child the following 5 activities in this order.
All activities are created by teachers and automatically marked. Plus, with an EdPlace subscription, we can automatically progress your child at a level that's right for them. Sending you progress reports along the way so you can track and measure progress, together - brilliant!
Activity 1 - Explaining Electrolysis Overall
Activity 2 - Explain Ionic Bonding
Activity 3 - Understand the Electrolysis of Solution
Activity 4 - Describe Electrolysis in Terms of Electron Transfer
Activity 5 - Revise Extracting Substances with Electrolysis
Answers
a) Copper (Cu2+) is a metal because it has a positive charge. All metal ions have a positive charge.
b) Chlorine (Cl-) is a non-metal because its ions have a negative charge. All non-metal ions have a negative charge.
c) Melting through heating or dissolving could break the bonds in copper chloride (CuCl2) to separate it into copper ions (Cu2+) and chloride ions (Cl-). This then allows the ions to move to the electrodes.
d) The copper ions (Cu2+) will move towards the negative electrode (cathode) when the electricity is turned on because opposite charges attract. Metals ions always have a positive charge and always move to the negative electrode.
Stretch and challenge:
e) When it moves to the negative electrode, the copper ion (Cu2+) will gain 2 electrons in a reduction reaction. Metals always gain electrons in reduction reactions.
f) When they move to the positive electrode the chlorine ion (Cl-) will lose 1 electron each in an oxidation reaction. Non-metals always lose electrons in oxidation reactions.
Keep going! Looking for more activities, different subjects or year groups?
Click the button below to view the EdPlace English, maths, science and 11+ activity library