Remember those tiny little things called atoms? They make up absolutely everything! So it's no surprise that they play the leading role in all chemical reactions and changes of state!

In both physical changes of state and chemical reactions, mass is conserved and the number of atoms stays the same. Atoms cannot be destroyed. This is known as the Law of Conservation of Mass.
.jpg)
Changes of state are a physical change. But the mass is always conserved, as well as the number of atoms involved. For example, if the mass of an ice cube in solid form was 100 g, then the mass of the water in liquid form would also be 100 g and the mass of the water vapour when it is in gaseous form would also be 100 g.
Chemical reactions are similar - although it involves a bit more maths sometimes, depending on how complex the chemical reaction is, to see that mass is conserved here too.
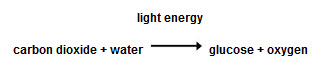
The reaction above is called photosynthesis. It's the way that plants make food for themselves using sunlight.
.jpg)
If we measured the mass of the reactants (carbon dioxide and water) before the reaction, and the mass of one of the products (the mass of oxygen produced), we could work out how much glucose has been produced for use by the plant.
Carbon dioxide + Water → Glucose + Oxygen
75 g + 14 g → + 20 g
75 + 14 = 89 (the mass of the reactants is 89 g)
89 - 20 = 69 g
So the mass of glucose produced for use by the plant is 69 g - lucky plant!
As you can see, the mass of the two substances before is the same as the mass of the products made!
The rule therefore is that the mass of the reactants equals the mass of the products!
It's all to do with atoms. When a chemical reaction takes place, atoms in a starting substance rearrange themselves and join back together to form a new substance. In a physical change, atoms rearrange themselves, either getting further apart or closer together. But, the number of atoms will still be the same before and after the reaction.
How do you feel about conservation of mass? Is it all coming together now?

Let's try some questions.








